Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber.
Example: Azobenzene
Dye Properties
§ Some remarkable properties are:
§ Dye molecules of the dye stuff are colored
§ Must contain chromophore group,
o which reacts as a color bearing group
§ Able to prepare dilute solution by dissolving water
§ Must have power of entering dye stuff to fiber from dye bath
§ Should have fastness properties
o i.e., color fastness to light, wash, rubbing, etc.
Pigments
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption.
This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.
Example: Quinacridone, Dioxazine
Pigment Properties
Some remarkable properties are:
§ Water insoluble
§ May be organic or inorganic in nature
§ Size of pigment particles are 1-2µ
§ Should have good covering power
§ Should have freely mixing properties
§ Should have good resistance to solvent
Sources of Dye
§ Vegetable and Animal sources
§ Turkey Red from root of Madder plant
§ Indigo (Blue) from leaves of Indigo plants
§ Saffron (Yellow) from the dried flower of color thistle
§ Mauveme - violate dye for silk. W.H. Parker prepared in 1856, which is first synthetic dye
§ Magenda - Synthesized in 1857
At present, most of dyes are synthetic; they are prepared from Benzene, Phenol, Aniline, Naphthalene, etc.
Printing
Printing involves localized coloration. This is usually achieved by applying thickened pastes containing dyes or pigments onto a fabric surface according to a given colour design. In particular, the viscosity of a print paste is critical. It determines the volume of paste transferred to the fabric and the degree to which it spreads on and into the surface yarns. The paste must colour all the visible fibres on the printed surface, so it must penetrate somewhat into the yarn structure. If the paste is too ‘thin’, it will spread, giving poor print definition, and penetrate too far into the yarns decreasing the colour yield.
The rapid development of CAD computer systems for print design has had a significant impact on this activity. The development of digitised textile printing using, for example, ink jet printers is well underway. Such computer assisted manufacturing will considerably influence the textile printing industry in the near future. Its other preoccupation, as for the dyeing industry in general, is that of reducing the amounts of biodegradable and potentially harmful chemicals in the effluent leaving the works so that its environmental impact is limited.
Basic Color Theory
Sir Isaac Newton developed the first circular diagram of colors in 1666. Since then, scientists and artists have studied and designed numerous variations of this concept.
Differences of opinion about the validity of one format over another continue to provoke debate. In reality, any color circle or color wheel which presents a logically arranged sequence of pure hues has merit.
Primary Colors: Red, yellow and blue
In traditional color theory (used in paint and pigments), primary colors are the 3 pigment colors that can not be mixed or formed by any combination of other colors. All other colors are derived from these 3 hues.
In traditional color theory (used in paint and pigments), primary colors are the 3 pigment colors that can not be mixed or formed by any combination of other colors. All other colors are derived from these 3 hues.
Secondary Colors: Green, orange and purple
These are the colors formed by mixing the primary colors.
Tertiary Colors: Yellow-orange, red-orange, red-purple, blue-purple, blue-green & yellow-green
These are the colors formed by mixing a primary and a secondary color. That's why the hue is a two word name, such as blue-green, red-violet, and yellow-orange.
These are the colors formed by mixing the primary colors.
Tertiary Colors: Yellow-orange, red-orange, red-purple, blue-purple, blue-green & yellow-green
These are the colors formed by mixing a primary and a secondary color. That's why the hue is a two word name, such as blue-green, red-violet, and yellow-orange.
 |
a color chart |
Different types of Dye
Acid dyes are water-soluble anionic dyes that are applied to fibers such as silk, wool, nylon and modified acrylic fibers using neutral to acid dye baths. Attachment to the fiber is attributed, at least partly, to salt formation between anionic groups in the dyes and cationic groups in the fiber. Acid dyes are not substantive to cellulosic fibers. Most synthetic food colors fall in this category.
Basic dyes are water-soluble cationic dyes that are mainly applied to acrylic fibers, but find some use for wool and silk. Usually acetic acid is added to the dye bath to help the uptake of the dye onto the fiber. Basic dyes are also used in the coloration of paper.
Direct or substantive dyeing is normally carried out in a neutral or slightly alkaline dye bath, at or near boiling point, with the addition of either sodium chloride (NaCl) or sodium sulfate (Na2SO4) or sodium carbonate (Na2CO3). Direct dyes are used on cotton, paper, leather, wool, silk and nylon. They are also used as pH indicators and as biological stains.
Vat dyes are essentially insoluble in water and incapable of dyeing fibers directly. However, reduction in alkaline liquor produces the water soluble alkali metal salt of the dye, which, in this leuco form, has an affinity for the textile fiber. Subsequent oxidation reforms the original insoluble dye. The color of denim is due to indigo, the original vat dye.
Mordant dyes require a mordant, which improves the fastness of the dye against water, light and perspiration. The choice of mordant is very important as different mordants can change the final color sign if therefore a large literature base describing dyeing techniques. The most important mordant dyes are the synthetic mordant dyes, or chrome dyes, used for wool; these comprise some 30% of dyes used for wool, and are especially useful for black and navy shades. The mordant, potassium dichromate, is applied as an after-treatment. It is important to note that many mordants, particularly those in the heavy metal category, can be hazardous to health and extreme care must be taken in using them
Reactive dyes utilize a chromophore attached to a substituent that is capable of directly reacting with the fibre substrate. The covalent bonds that attach reactive dye to natural fibers make them among the most permanent of dyes. "Cold" reactive dyes, such as Procion MX, Cibacron F, and Drimarene K, are very easy to use because the dye can be applied at room temperature. Reactive dyes are by far the best choice for dyeing cotton and other cellulose fibers at home or in the art studio.
Disperse dyes were originally developed for the dyeing of cellulose acetate, and are water insoluble.
The dyes are finely ground in the presence of a dispersing agent and sold as a paste, or spray-dried and sold as a powder. Their main use is to dye polyester but they can also be used to dye nylon, cellulose triacetate, and acrylic fibres. In some cases, a dyeing temperature of 130 °C (266 °F) is required, and a pressurized dye bath is used. The very fine particle size gives a large surface area that aids dissolution to allow uptake by the fibre. The dyeing rate can be significantly influenced by the choice of dispersing agent used during the grinding.
Azoic dyeing is a technique in which an insoluble azo dye is produced directly onto or within the fibre. This is achieved by treating a fibre with both diazoic and coupling components. With suitable adjustment of dye bath conditions the two components react to produce the required insoluble azo dye. This technique of dyeing is unique, in that the final color is controlled by the choice of the diazoic and coupling components. This method of dyeing cotton is declining in importance due to the toxic nature of the chemicals used.
Sulfur dyes are two part "developed" dyes used to dye cotton with dark colors. The initial bath imparts a yellow or pale chartreuse color, this is after treated with a sulfur compound in place to produce the dark black we are familiar with in socks for instance. Sulfur Black 1 is the largest selling dye by volume.
Types of Printing
Types of Printing
Pigment printing
Pigment printing has gained much importance today and for some fibers (e.g. cellulose fibers) is by far the most commonly applied technique. Pigments can be used on almost all types of textile substrates and it is now possible to obtain high-quality printing using this technique.
Pigment printing pastes contain a thickening agent, a binder and other auxiliaries such as fixing agents, plasticizers, defoamers.
Discharge printing
In this approach, the fabric is dyed in piece and then it is printed with a chemical that destroys the colour in the designed areas. Sometimes, the base colour is removed and another colour is printed in its place. The printed fabric is steamed and then thoroughly washed. This approach is on decline these days.
Reactive printing
Reactive printing is a method of printing a dye or wax by using mixes thereof to create colors. With a binder and a heat-activated printing additive, images can be permanently bonded to the substrate (typically textiles, but can include cellulose, fibers, polyester, and even proteins). These reactions are generally heat-activated.
Sensation of Color
Ordinary light is composed of reds of ray of different wave lengths (visible wave length of 4000 to 8000 Ao)
§ If all bonds reflects, substance appear white. In case of absorbs, it’s black
§ In case of definite wave length’s reflection, the corresponding color will be visible
§ In case of absorbing any single bond and reflecting rest of bonds, that’s composite color and gives a shade.
Chromophore
Derived from Greek word “Chroma” means “Color” and “Phore” means “to bear”.
So, Chromophore is the color bearing group
Auxochromo derived from Greek word “Auxanien” means “to intensify” and “Chroma” means “Color”. So, the color intensifying groups are Auxochromo
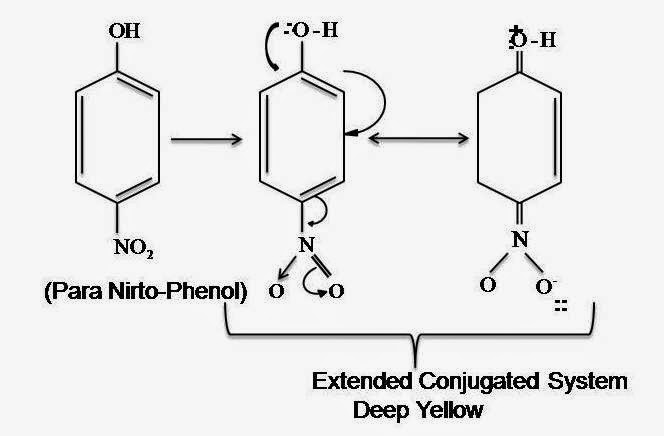
Modern Conjugation Theory
Long conjugation enables the p electrons to delocalize throughout the system to decrease energy gaps between molecular orbits in the ground state & molecular in the excited state
The longer conjugation helps to excite easily to de-localize p electrons & show the color.
Auxochromo intensify the color of Chromophore by creating extended conjugated systems.
Here, Hydroxyle group (-OH) is an Auxochromo
Nomenclature
A publication “Color Index International” by the Society of Dryers and Colorist of the UK and the American Association of Textile Chemists & Colorist–
“Dye stuff are classified based on their chemical structure (CI Constitution Number) & according to the application or usage (CI Generic Name) ”
CI Constitution Number
- Based on chemical structure of the dye, which contains chromophore (color producing group, i.e., Azo, Antraquinone, Nitro etc.)
- There is an individual number assigned for each dye which consists five digits each
- The numbering starts from 10,000. For example, the first 300 numbers are assigned to Nitro dyes (10001 - 10300)
- If in future, any new variation dye stuff is being introduced, the intermediate is assigned to it
CI Generic Name
- Dye stuffs are classified according to their usage & the stuff are subdivided depending on their hue
- These are grouped as Acid, Basic, Direct, Disperse, Reactive, Mordant, Pigment, Sulpher, Vat dyes, etc. and subdivided as Yellow, Orange, Red, Violet, Blue, Green, Brown, Black, etc.
- Secondary division of the dyes from each primary division class is given consecutive number.
- Example, there are 387 Red dyes under Acid Dyes & these are denoted as CI Acid Red 1 to CI Acid Red 387
Nomenclature of Commercial Dyes
Certain letters appears after the words indicative of the color of the dye. This requires the tone of the color.
R = Reddish tone (Red, German word Rot)
G = Yellowish tone (Yellow, German word Gèlb)
B = Bluish tone (Blue, German word Blèu)
When the dye produces a shade on fiber & is fast to light, the letter “L” appears in the name
Example:
Classification of Dye
Dyeing Parameter Control Chart
§ Water level before fabric loading(Lit)
§ Loading Time(min)
§ Water level after fabric loading(after running 05 min)
§ Dosing time of caustic
§ Run time
§ Dosing time of per-oxide
§ Total time need to raise temp of 105ºC
§ Gradient (Degree/min)
§ Total run time(min)
§ Total time need to cooling at 80c
§ Total MIR /Rinsing time to 50c
§ Total run time after acid dosing
§ Check PH
§ Total runtime after peroxide killer dosing
§ Check residual per-oxide (ppm)
Pollutants during printing process
Drying and fixing are another important emission source in printing processes. The following pollutants may be encountered in the exhaust air [179, UBA, 2001]:
- aliphatic hydrocarbons (C10-C20) from binders
- monomers such as acrylates, vinylacetates, styrene, acrylonitrile, acrylamide, butadiene
- methanol from fixation agents
- other alcohols, esters, polyglycols from emulsifiers
- formaldehyde from fixation agents
- ammonia (from urea decomposition and from ammonia present, forexample, in pigment printing pastes)
- N-methylpyrrolidone from emulsifiers
- phosphoric acid esters
- phenylcyclohexene from thickeners and binders













No comments:
Post a Comment